When you pick up a prescription for generic sertraline or metformin, you’re probably paying under $10. That’s not luck. It’s the result of a deliberate, decades-long system designed to let competition, not government officials, set the price. Unlike branded drugs, where companies hold patents and charge high prices to recoup research costs, generic drugs enter a market flooded with competitors. And that’s exactly how governments want it.
Why Generic Drugs Don’t Need Price Caps
Generic drugs aren’t cheaper because the government said so. They’re cheaper because dozens of companies are fighting to sell them. After a brand-name drug’s patent expires, any manufacturer can apply to the FDA to make an identical version. They don’t need to repeat expensive clinical trials. They just need to prove their pill works the same way-bioequivalence. That cuts development costs from $2.6 billion to about $2-3 million. The result? Prices drop fast.By the time a generic hits the market, the original brand might still be priced at $150 a month. Within six months, a single generic version brings it down to $35. By two years, if three or more companies are selling it, the price often falls to $5 or less. In 2022, the FDA found that when five or more generics compete, prices drop 90% from the brand’s original price. That’s not regulation. That’s capitalism working as intended.
The U.S. government doesn’t set these prices. It removes barriers so competition can. The Hatch-Waxman Act of 1984 created the Abbreviated New Drug Application (ANDA) pathway. It didn’t cap prices-it opened the door. And since then, generics have made up 90% of all prescriptions filled in the U.S., while accounting for only 23% of total drug spending. That’s the power of competition.
How the FDA Speeds Up Generic Approvals
The real government tool isn’t a price ceiling-it’s speed. The FDA’s Generic Drug User Fee Amendments (GDUFA), renewed in 2022 with $750 million in industry fees, was built to cut approval times. Before GDUFA, it took 18 months on average to approve a generic. Now, the goal is 10 months. In 2023, the FDA approved 1,083 generic drugs-the highest number in a decade. That’s not random. It’s a target.They’ve hit their mark: 92% of priority generic applications got a decision within 10 months in 2023. For complex generics-drugs with tricky delivery systems like inhalers or injectables-it’s harder. Only 38% met the 10-month target. So in late 2023, the FDA launched a new submission template to help manufacturers navigate those complex cases. Pilot programs using the template cut review times by 35%.
The FDA also tracks every application publicly through its Generic Drug User Fee Public Dashboard. You can see exactly where a drug is in the process-submitted, under review, approved. Transparency keeps pressure on the agency and helps manufacturers plan. No backroom deals. No delays hidden from view.
Stopping Anti-Competitive Tactics
But competition doesn’t always win on its own. Sometimes, brand-name companies pay generic makers to delay entry. These are called "pay-for-delay" deals. In 2023, the Federal Trade Commission (FTC) challenged 37 of them. These agreements kept generics off the market for years, letting brands charge high prices. The FTC estimates that breaking just these 37 deals could save consumers $3.5 billion a year.The FTC also blocked the proposed merger between Teva and Sandoz in January 2024. Together, they controlled 30% of the U.S. generic market. The agency said the merger would reduce competition for 13 key drugs, including antibiotics and blood pressure meds. That’s not about controlling prices-it’s about preserving the conditions where prices naturally fall.
Even more, the FTC and the Department of Justice have started targeting "product hopping"-when a brand makes a minor change to its drug (like switching from a pill to a capsule) and then pushes patients to the new version, effectively blocking generics. The FDA’s 2024-2026 plan now explicitly targets these tactics, encouraging authorized generics to enter the market early and keep prices low.

Why Medicare Doesn’t Negotiate Generic Prices
The Inflation Reduction Act of 2022 let Medicare negotiate prices for 10 high-cost brand-name drugs in 2026, expanding to 15 in 2027. But generics? Excluded. Why?The Department of Health and Human Services made it clear: generics already have competition. The law only lets Medicare negotiate for drugs with no generic or biosimilar alternatives. In April 2024, CMS confirmed that applying negotiation to generics would save only $1.2 billion a year-less than 0.5% of total generic spending. Meanwhile, negotiating just 15 branded drugs like Ozempic and Wegovy could save $9.5 billion.
The Congressional Budget Office found the same thing. Applying international price benchmarks to generics would cut Medicare spending by just $2.1 billion annually. For branded drugs? $158 billion. That’s why policymakers don’t waste time on generics. The market already does the job better.
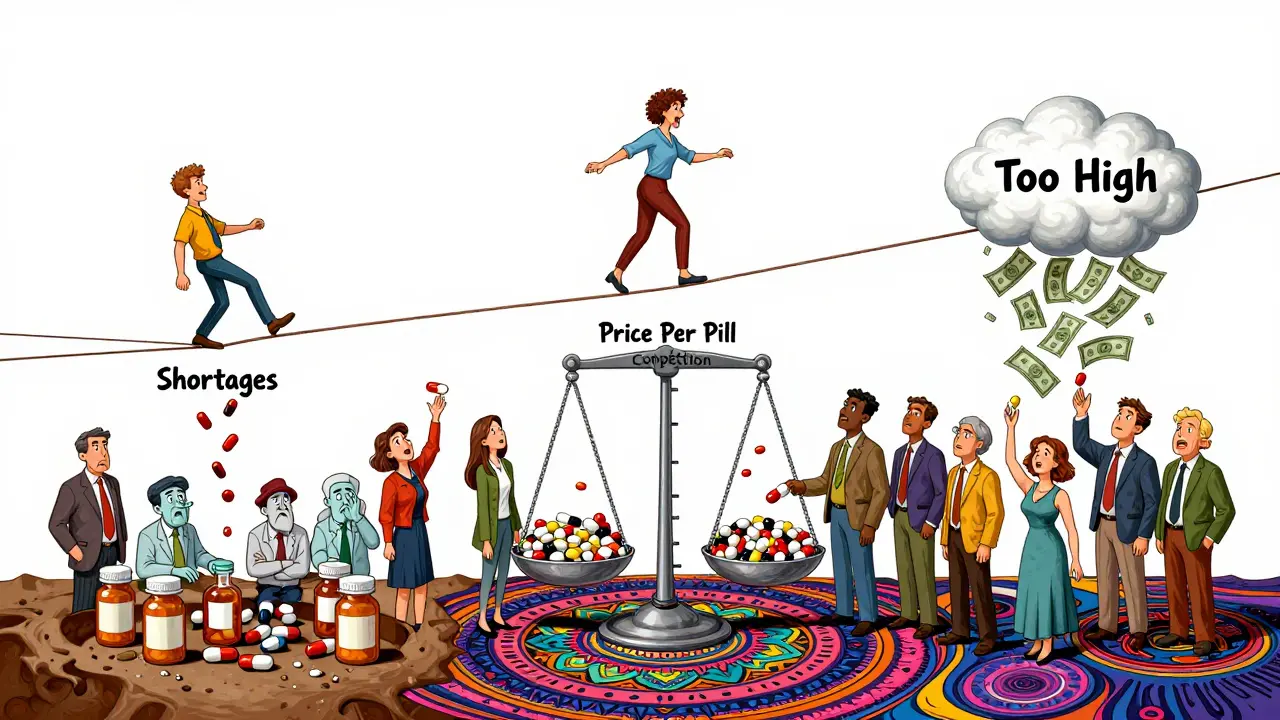
What Happens When Prices Drop Too Low?
There’s a flip side. When prices fall too far, manufacturers stop making the drug. In 2023, the FDA reported 155 drug shortages, and 82% were for generic medications. Why? Because the price per pill fell below the cost of production.A 2024 survey by the American Society of Health-System Pharmacists found that 18% of hospital pharmacists had run out of critical generics like doxycycline or furosemide because manufacturers discontinued them. Forty-three percent said prices had dropped so low they couldn’t cover manufacturing costs.
It’s a tightrope. Too high, and patients can’t afford it. Too low, and the drug disappears. That’s why the FDA and FTC focus on keeping the market fair-not setting prices. They want enough competition to drive prices down, but not so much that no one can profitably make the drug.
What Patients Actually Pay
For most people, generic drugs are a win. The 2024 KFF Consumer Survey found that 76% of Medicare Part D users paid $10 or less for their generic prescriptions. Only 28% paid that little for brand-name drugs. And 82% of generic users said they were satisfied with affordability. Compare that to 41% for brand-name users.On Drugs.com, 87% of reviews for generic drugs mentioned "affordable" or "cost-effective" as the top reason they chose the drug. Only 5% raised pricing concerns.
But there are outliers. One Reddit user in April 2024 reported their generic sertraline jumped from $4 to $45 a month. That wasn’t a nationwide trend. The FDA’s Drug Shortage Report showed that only 0.3% of generics had price spikes like that. Those are usually caused by supply chain issues or temporary monopolies-not systemic failure.
The Bigger Picture: U.S. vs. the World
The U.S. has the most competitive generic market in the world. On average, there are 14.7 manufacturers per generic drug here. In Europe, it’s 8.2. In Japan, it’s 5.3. That’s why U.S. generic prices are lower, even though we spend more overall on drugs.The U.S. makes up 42% of global generic consumption by volume but only 29% by value. That means we buy more pills, but pay less per pill. Other countries use direct price controls. The U.S. uses competition. And it works.
The global generic market is growing at 7.8% a year-faster than branded drugs. The U.S. leads that growth because it lets companies compete freely. Any attempt to impose price caps would likely slow down approvals, reduce manufacturer interest, and lead to more shortages.
What’s Next for Generic Pricing?
The focus now isn’t on setting prices. It’s on removing roadblocks. The CMS Interoperability and Prior Authorization Proposed Rule, issued in April 2024, aims to stop insurance plans from requiring unnecessary prior authorizations for generics. That could save patients $420 million a year.The Generic Pharmaceutical Association’s Competitive Generic Therapy (CGT) designation gives faster review to generics entering markets with little competition. That’s a smart tool-targeted, not broad.
The Congressional Budget Office projects generic drug prices will keep falling at 3.5% per year through 2030. Branded drugs? Only 0.8%. That gap isn’t accidental. It’s the result of policies that protect competition, not interfere with it.
Government doesn’t need to control generic prices. It just needs to keep the playing field level. And right now, that’s exactly what it’s doing.
Why don’t governments set prices for generic drugs like they do for branded ones?
Governments don’t set prices for generics because competition already drives them down. When multiple companies make the same drug, prices fall naturally-often to 10-15% of the original brand price. Direct price controls would risk reducing manufacturer incentives, leading to shortages. The FDA and FTC focus instead on removing barriers to entry and blocking anti-competitive behavior.
Can generic drug prices ever go up?
Yes, but rarely and usually for specific reasons. Price spikes happen when only one or two manufacturers make a drug, or when there’s a shortage due to manufacturing issues or raw material problems. In 2023, only 0.3% of generics saw significant price increases. Most spikes are temporary and localized, not systemic. The FDA tracks these cases and works with manufacturers to restore supply.
Do generic drugs work the same as brand-name drugs?
Yes. The FDA requires generics to be bioequivalent to the brand-name version-meaning they deliver the same amount of active ingredient into the bloodstream at the same rate. They may have different fillers or coatings, but the active ingredient and effectiveness are identical. Over 90% of U.S. prescriptions are filled with generics because they’re proven safe and effective.
Why do some hospitals run out of generic drugs?
When the price per pill drops below the cost to make and distribute it, manufacturers stop producing it. This happened with drugs like doxycycline and furosemide. In 2024, 18% of hospital pharmacists reported shortages due to unsustainable pricing. The issue isn’t demand-it’s profit. The FDA and FTC are working to prevent this by encouraging more manufacturers to enter the market and blocking anti-competitive practices.
Is the U.S. system better than other countries’ for generic drug pricing?
In terms of volume and affordability, yes. The U.S. has more manufacturers per generic drug than any other developed country-14.7 on average, compared to 8.2 in Europe and 5.3 in Japan. That competition keeps prices low. Other countries use direct price controls, but often end up with fewer choices and longer wait times. The U.S. model prioritizes access and speed, and it works: 90% of prescriptions are generics, and most cost under $10.


Gregory Parschauer
January 14, 2026 AT 12:08Let me just say this: the idea that ‘competition fixes everything’ is the most dangerous myth in modern healthcare. You think 14 manufacturers of doxycycline means ‘fair pricing’? Nah. It means they’re all racing to the bottom until someone drops out-and then the remaining few jack prices up because patients have zero alternatives. This isn’t capitalism-it’s predatory deregulation dressed up as ‘market efficiency.’ And now hospitals are rationing antibiotics because no one can profitably make a $0.02 pill. Wake up.
Alan Lin
January 15, 2026 AT 19:23While I appreciate the structural analysis presented here, I must respectfully challenge the underlying assumption that market competition alone is sufficient to ensure equitable access. The data on drug shortages, while statistically small in percentage terms, represents a profound human cost: elderly patients unable to obtain essential medications, emergency departments forced to substitute less effective agents, and clinicians making life-altering decisions based on inventory logs rather than clinical guidelines. The FDA’s transparency dashboard is commendable, yet it does not address the fundamental asymmetry between corporate profit motives and public health imperatives.
Moreover, the exclusion of generics from Medicare negotiation is not merely a technicality-it is a moral failure disguised as economic pragmatism. A $1.2 billion savings may seem trivial against $9.5 billion, but for the 12 million Medicare beneficiaries who rely on those generics, that $1.2 billion is the difference between adherence and abandonment. Policy must account for human dignity, not just aggregate cost curves.
Vinaypriy Wane
January 16, 2026 AT 19:08Diana Campos Ortiz
January 18, 2026 AT 15:39Jesse Ibarra
January 20, 2026 AT 01:55Oh, so now we’re celebrating corporate deregulation as ‘capitalism working’? Please. The FDA isn’t a neutral referee-it’s a captive agency funded by the very industry it’s supposed to regulate. GDUFA fees? That’s not transparency, it’s regulatory capture. And don’t get me started on the FTC blocking mergers-because they only act when the public outcry gets loud enough. Meanwhile, the same pharma giants that pay for generics to delay entry are the ones lobbying Congress to block Medicare negotiation. This isn’t a free market. It’s a rigged game where the house always wins-and patients pay the price in suffering.
And let’s not pretend ‘14 manufacturers’ means choice. Most of them are subsidiaries of the same 3 mega-corporations. You think Teva and Sandoz aren’t playing the same game? They just hide behind different names. This whole narrative is a distraction. Real reform means breaking up the oligopoly-not patting ourselves on the back for ‘low prices’ while people die from insulin rationing.
laura Drever
January 21, 2026 AT 01:22Randall Little
January 23, 2026 AT 00:27So let me get this straight: the U.S. government doesn’t set prices… but it sets approval timelines, funds industry fees to speed things up, blocks mergers, targets product hopping, and publicly tracks every submission… all to ‘protect competition.’
That’s not laissez-faire. That’s hyper-managed capitalism with a PR team. You call it ‘removing barriers.’ I call it ‘orchestrating the market like a symphony with a billion-dollar conductor.’
And yet you’re shocked when prices drop so low that pills vanish? Of course they do. You didn’t leave the market alone-you built a machine designed to crush margins until the last manufacturer quits. Then you blame ‘the market’ for failing. Classic.
Meanwhile, Canada and the UK just say, ‘We’ll pay $X.’ And guess what? No shortages. No drama. Just pills. Maybe we should try that.
jefferson fernandes
January 24, 2026 AT 23:17Look, I get it-competition sounds nice on paper. But let’s talk about real people. My cousin takes sertraline. She’s on Medicare. She pays $5 a month. That’s great. But last year, her pharmacy ran out. For three weeks. No one told her why. No one called to say, ‘Hey, the manufacturer shut down production because the price per pill was 3 cents and the shipping cost was 4.’
She had to switch to the brand-name version. Cost: $420. She cried. She skipped doses. She lost her job for a month because her depression spiked.
So yeah, the system ‘works’ for the 99% of people who don’t hit the 0.3% glitch. But for the 1% who do? The system is a death sentence. And we’re not fixing it. We’re just writing blog posts about how ‘competition is beautiful.’
Real leadership means ensuring no one falls through the cracks. Not just optimizing for cost per pill. Not just tracking FDA timelines. We need safety nets. We need buffer stockpiles. We need incentives for manufacturers to keep making the cheap stuff-even if the profit’s thin. Because human lives aren’t a spreadsheet.